- Patients Patients
Reproductive Genetics Testing
Patient Resources
Cost & Billing
- Providers Providers
- Genetic Counseling
- Login Login
- Estimate My Cost

Comprehensive preeclampsia Testing
Preeclampsia screening across all pregnancy trimesters
Preeclampsia symptoms are similar to common pregnancy-related symptoms, which often leads to delayed preeclampsia diagnosis and intervention. Now with Labcorp’s preeclampsia screening during first, second and third trimesters, identifying those at risk and enhancing management of high-risk pregnancies can help lead to earlier detection and intervention and help reduce preeclampsia related complications and patient mortality.
Early screening can save lives
Up to 60% of preeclampsia-related deaths are preventable1, making early detection critical. Common symptoms of preeclampsia include: persistent headache, blurry vision, seeing spots, or having changes in eyesight, pain in the upper stomach area, swelling of the face or hands and sudden weight gain.2
Comprehensive solutions for preeclampsia detection
1st Trimester Preeclampsia Screen
Labcorp’s first trimester blood test can help determine a pregnant patient’s risk for developing preeclampsia early in pregnancy before the patient typically becomes symptomatic (approximately 20 weeks’ gestation).
Second / Third Trimester Preeclampsia Tes
To aid in the risk assessment of pregnant women hospitalized for hypertensive disorders of pregnancy for progression to preeclampsia with severe features within 2 weeks of presentation.
Clinical studies on preeclampsia screening and testing,
treatment, and its economic impact.
Identify those at high risk and, as needed, enable earlier intervention
Similar to common pregnancy symptoms, early signs of preeclampsia can include a rise in blood pressure, swelling, protein in the urine, changes in vision, nausea and headache. First trimester preeclampsia screening at 11-14 weeks’ gestation can help you avoid missing the window for early detection and intervention.
Enhance clinicians’ management of high-risk pregnancies
When administered early, prophylactic aspirin treatment may help reduce the risk and severity of preeclampsia3 and allow for a decision to be made on whether a patient may need additional care including possible referral to a larger health center or tertiary care facility.
Reduce the economic burden for both mother and baby
With an increased risk of preterm births, NICU stays and other complications,4 the estimated total cost burden of preeclampsia during the first 12 months after birth is $2.18 billion for infants.5 Early preeclampsia screening can help reduce the overall financial burden to the healthcare system by reducing preterm births and NICU admissions or durations.
Easy ordering and reporting
Read the ASPRE and SPREE studies highlighting various approaches to the first-trimester preeclampsia screening and their respective detection rates.
Read the PRAECIS study13 published in NEJM Evidence that highlights second and third trimester preeclampsia testing for pregnant women hospitalized for a hypertensive disorder in a multicenter U.S. study.
Preeclampsia screening during first, second, and third trimesters
Labcorp's first-trimester preeclampsia screening test uses a blood specimen drawn between 11 to 14 weeks' gestation, a combination of maternal factors, biochemical markers (PlGF and PAPPA), and biophysical markers (MAP and UtAPI), along with a proprietary algorithm to assess the risk of developing preeclampsia later in pregnancy (<34 weeks' gestation). This screening test is suitable for low- and high-risk patients with singleton or twin gestation pregnancies.
In the second and third trimesters, a blood draw between 23.0 to 34.9 weeks' gestation uses two biomarkers (sFlt-1 and PlGF) to assess the risk of preeclampsia progressing to severe features within the subsequent two weeks. This test is FDA cleared for use in hospitalized patients (singleton pregnancies).
Table 1. Preeclampsia testing features
| First Trimester Preeclampsia Screen | Second/Third Trimester Preeclampsia Test | |
|---|---|---|
| Intended use | Screening test – Risk of developing preeclampsia prior to 34 weeks' gestation | Prognostic confirmation test – Risk of preeclampsia progressing to severe features within 2 weeks gestation |
| Blood draw | First trimester (11 – 14 weeks’ gestation) | Second & third trimesters (23 – 34.9 weeks’ gestation) |
| Regulatory category | Laboratory developed test (LDT) | FDA cleared |
| Clinical application | Low- and high-risk patients with singleton or twin gestation pregnancies | Hospitalized symptomatic (hypertensive disorder) patients with singleton gestation pregnancies |
| Specimen type | Serum, frozen or refrigerated | Serum or plasma (K2 EDTA), frozen |
| Clinical data | UK (SPREE & ASPRE) | U.S. (PRAECIS) |
| Results | Individualized risk (i.e. 1:150) with a cutoff of 1:100 for high risk | sFlt-1:PlGF ratio (≥40 = High risk) |
| TAT | 2-5 days | 1-2 days |
| Biomarkers | Biochemical markers: PlGF = Placental growth factor (protein) PAPP-A = Pregnancy associated plasma protein A (protein) Biophysical markers: MAP = Mean arterial pressure (dual-cuff blood pressure monitor) UtAPI = Uterine artery pulsatility index (transvaginal ultrasound/doppler) | sFlt-1 = Serum soluble fms-like tyrosine kinase 1 (protein) PlGF = Placental growth factor (protein) |
| Aneuploidy | n/a | n/a |
| Intended Use | |
|---|---|
| First Trimester Preeclampsia Screen | Screening test – Risk of developing preeclampsia prior to 34 weeks' gestation |
| Second/Third Trimester Preeclampsia Test | Diagnostic confirmation test – Risk of preeclampsia progressing to severe features within 2 weeks |
| Blood draw | |
|---|---|
| First Trimester Preeclampsia Screen | First trimester (11 – 14 weeks’ gestation) |
| Second/Third Trimester Preeclampsia Test | Second & third trimesters (23– 34.9 weeks’ gestation) |
| Regulatory category | |
|---|---|
| First Trimester Preeclampsia Screen | Laboratory developed test (LDT) |
| Second/Third Trimester Preeclampsia Test | FDA cleared |
| Clinical application | |
|---|---|
| First Trimester Preeclampsia Screen | Any pregnant patient |
| Second/Thrd Trimester Preeclampsia Test | Hospitalized symptomatic (hypertensive disorder) patients |
| Specimen type | |
|---|---|
| First Trimester Preeclampsia Screen | Serum, frozen or refrigerated |
| Second/Third Trimester Preeclampsia Test | Serum or plasma (K2 EDTA), Frozen |
| Clinical data | |
|---|---|
| First Trimester Preeclampsia Screen | UK (SPREE & ASPRE) |
| Second/Third Trimester Preeclampsia Test | U.S. (NEJM) |
| Results | |
|---|---|
| First Trimester Preeclampsia Screen | Individualized risk (i.e. 1:150) with a cutoff of 1:100 for high risk |
| Second/Third Trimester Preeclampsia Test | sFlt-1:PlGF ratio (≥40 = High risk) |
| TAT | |
|---|---|
| First Trimester Preeclampsia Screen | 2-5 days |
| Second/Third Trimester Preeclampsia Test | 1-2 days |
| Biomarkers | |
|---|---|
| First Trimester Preeclampsia Screen | Biochemical markers: PlGF = Placental Growth Factor (protein) PAPP-A = Pregnancy associated plasma protein A (protein) Biophysical markers: MAP = Mean arterial pressure (dual-cuff blood pressure monitor) UtAPI = Uterine artery pulsatility index (transvaginal ultrasound/doppler) |
| Second/Third Trimester Preeclampsia Test | sFlt-1 = Serum soluble fms-like tyrosine kinase 1 (protein) PlGF = Placental Growth Factor (protein) |
| Aneuploidy | |
|---|---|
| First Trimester Preeclampsia Screen | n/a |
| Second/Third Trimester Preeclampsia Test | n/a |
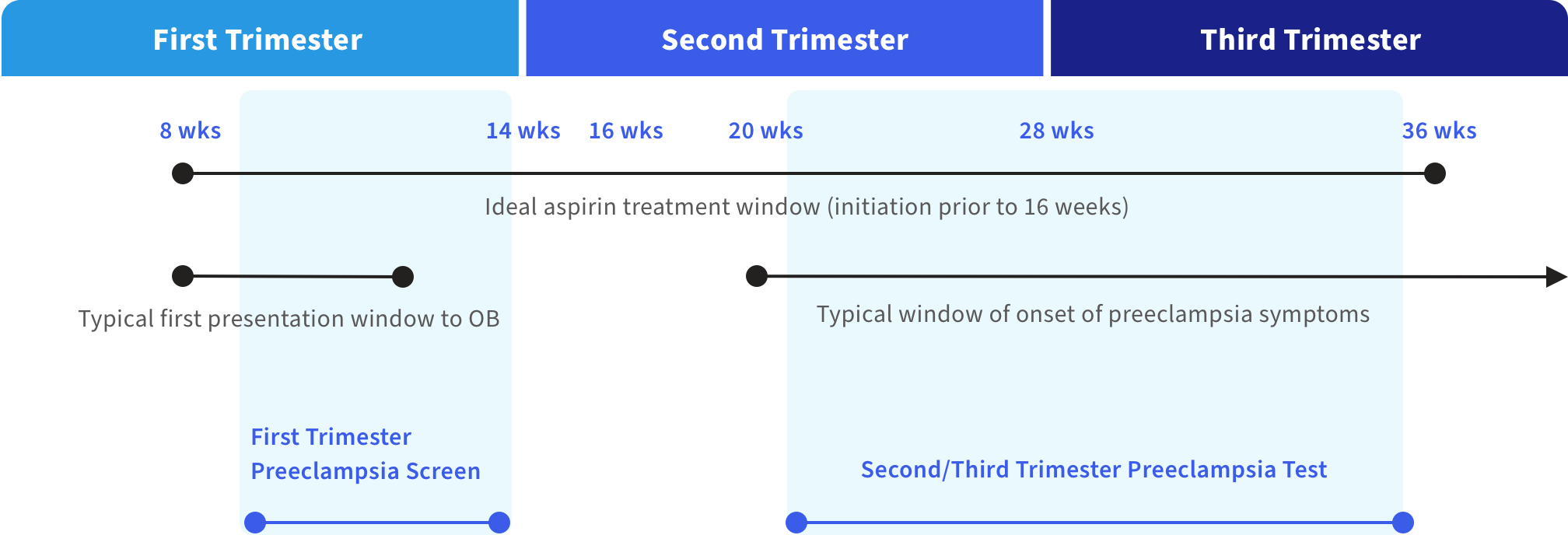
Figure 1. Preeclampsia testing, onset and treatment during pregnancy6
The biology behind preeclampsia is complex. Labcorp can help improve patient outcomes.
While the biology behind preeclampsia is complex, the leading causes are believed to be multifactorial, involving placental dysfunction and the failure to establish a robust blood vessel network within the placenta connecting mother and baby.
In an attempt to detect a patient’s risk of developing preeclampsia later in pregnancy, some screening approaches found in clinical practice today use maternal factors only. Others use one or more biochemical factors, while some newer approaches employ a combination of both in their risk assessment. Recent clinical research indicates that using maternal factors in combination with multiple biochemical and biophysical markers increase screening detection rate significantly.
Labcorp’s first-trimester preeclampsia screening test uses a combination of maternal factors plus two biochemical markers (PlGF and PAPP‑A) and two biophysical markers (MAP and UtAPI*) delivering test sensitivity of up to 90%,7 compared to less than 50% sensitivity from using maternal history and demographics alone.
*Sonographer training by The Fetal Medicine Foundation (FMF) or other equivalent entity is required for submission of UtAPI measurements. For more information around sonography certification please visit fetalmedicine.org.
Table 2. Various approaches to using maternal and biochemical factors, as well as biophysical markers in first-trimester preeclampsia risk assessment7
| Factors used in preeclampsia risk assessment | Sensitivity | Specificity |
|---|---|---|
| Maternal factors, PlGF, PAPP-A | 68.3% (55.0-79.7) | 90% |
| Maternal factors, PlGF, PAPP-A, MAP | 76.7% (64.0-86.6) | 90% |
| Maternal factors, PlGF, PAPP-A, UtAPI | 78.3% (65.8-87.9) | 90% |
| Maternal factors, PlGF, PAPP-A, UtAPI, MAP** | 90.0% (79.5-96.2) | 90% |
**Consistent with Labcorp’s approach to early preeclampsia risk assessment
| Factors used in preeclampsia risk assessment | |
|---|---|
| Maternal factors, PlGF, PAPP-A | |
| Sensitivity | 68.3% (55.0-79.7) |
| Specificity | 90% |
| Maternal factors, PlGF, PAPP-A, MAP | |
| Sensitivity | 76.7% (64.0-86.6) |
| Specificity | 90% |
| Maternal factors, PlGF, PAPP-A, UtAPI | |
| Sensitivity | 78.3% (65.8-87.9) |
| Specificity | 90% |
| Maternal factors, PlGF, PAPP-A, UtAPI, MAP* | |
| Sensitivity | 90.0% (79.5-96.2) |
| Specificity | 90% |
Labcorp’s second and third trimester preeclampsia test uses two biomarkers (sFlt-1 and PlGF) to assess the risk of preeclampsia progressing to severe features within the subsequent two weeks with test sensitivity up to 93.5%.
Table 3. Clinical performance of 2nd/3rd Trimester Preeclampsia Test13
| N=556 | Sensitivity*** | Specificity*** | PPV*** | NPV*** |
|---|---|---|---|---|
| Preeclampsia with severe features within 2 weeks | 93.5% (89.1 - 96.3) | 74.9% (70.2 – 79.0) | 65.2% (59.3 – 70.6) | 95.8% (92.9 – 97.6) |
| Preeclampsia with severe features within 2 weeks | |
|---|---|
| N=556 | |
| Sensitivity*** | 93.5% (89.1 - 96.3) |
| Specificity*** | 74.9% (70.2 – 79.0) |
| PPV*** | 65.2% (59.3 – 70.6) |
| NPV*** | 95.8% (92.9 – 97.6) |
***95% CI
Additional resources for preeclampsia awareness and education
Education
Helping to detect preeclampsia across all pregnancy trimesters
SMFM 2024
Community
Preeclampsia Foundation
Social change
Fund II Foundation
Press
CNN coverage of Labcorp’s first trimester preeclampsia test
Thought leadership
Webinar: Solving the puzzle of preeclampsia
Publication
Angiogenic biomarkers in preeclampsia
Still have questions?
Contact us or call 800-848-4436
References:
- Main EK, McCain CL, Morton CH, et al. Pregnancy-related mortality in California: causes, characteristics, and Improvement opportunities. Obstet Gynecol. 2015;125(4):938-947.
- Centers for Disease Control and Prevention. High Blood Pressure During Pregnancy. Accessed July 31, 2023. https://www.cdc.gov/bloodpressure/pregnancy.htm.
- ACOG Practice Advisory. Low-Dose Aspirin Use for the Prevention of Preeclampsia and Related Morbidity and Mortality. Accessed June 16, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/12/low-dose-aspirin-use-for-the-prevention-of-preeclampsia-and-related-morbidity-and-mortality.
- Chatzakis C, Liberis A, Zavlanos A, et al. Early delivery or expectant management for late preterm preeclampsia: A meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2021;100(8):1392-1400.
- Stevens W, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237-248.e16.
- Internal
- Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51(6):743-750. doi:10.1002/uog.19039.
- National Health Service. Pre-eclampsia - Causes. Accessed August 10, 2023. https://www.nhs.uk/conditions/pre-eclampsia/causes/.
- March of Dimes. Preeclampsia. Accessed August 10, 2023. https://www.marchofdimes.org/find-support/topics/pregnancy/preeclampsia.
- Penn Medicine Women’s Health Blog. The Warning Signs of Preeclampsia, Before and After Delivery. Accessed August 11, 2023. https://www.pennmedicine.org/updates/blogs/womens-health/2018/may/the-warning-signs-of-preeclampsia-before-and-after-pregnancy.
- Bateman BT, Shaw KM, Kuklina EV, et al. Hypertension in women of reproductive age in the United States: NHANES 1999-2008. PLoS One. 2012; 7(4): e36171. 2012 Apr 30. doi: 10.1371/journal.pone.0036171.
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin 222, June 2020. Obstet Gynecol. 2020 Jun; 135(6):e237-e260.
- Thadhani R, Lemoine E, Rana S, et al. Circulating Angiogenic Factor Levels in Hypertensive Disorders of Pregnancy. NEJM Evid. 2022;1(12). doi: 10.1056/EVIDoa2200161



